「领域快报」是AiBrain筹备的特别栏目,是由海内外知名高校的一线青年科研工作者(博士后、PI)精选的领域科研动态,旨在为学科融合、交叉合作提供平台和机遇。
「领域快报」胶质细胞
Science
脊髓小胶质细胞群参与神经性疼痛的缓解和复发
神经性疼痛通常是由神经系统的损伤和躯体感觉系统改变引起的疾病。这些改变发生在神经元和非神经元细胞中,尤其是小胶质细胞(一类组织定居的免疫细胞)。在周围神经损伤模型中,脊髓背角的小胶质细胞会发生快速的响应,并在形态、细胞数量和基因表达水平发生改变。小胶质细胞是一种高度可塑性的细胞,以不同的形态存在,具有高度的异质性,发挥着不同的功能。虽然疼痛的发病机制与小胶质细胞的关系已取得部分的研究成果,但小胶质细胞在疼痛恢复的机制仍然知之甚少。
Makoto Tsuda团队于2022年4月1日在国际顶级期刊《Science》(IF=47.728)发表研究性论文“A spinal microglia population involved in remitting and relapsing neuropathic pain”,发现了一种特殊类型的小胶质细胞与神经性疼痛缓解和复发的关系。

Science (IF 47.728)
★

本文通讯作者Makoto Tsuda,日本九州大学教授,他们长期专注于神经科学,神经性疼痛,神经损伤,小胶质细胞和周围神经损伤等研究。该实验室发表一系列与小胶质细胞相关的研究,其中包括转录因子MafB有助于在神经性疼痛发展的基础上激活脊髓小胶质细胞;小胶质细胞介导的神经性疼痛调节的分子和细胞机制;小胶质细胞释放的BDNF引起神经性疼痛下的神经元机制研究;在神经性疼痛方面的研究整合了嘌呤能受体和慢性疼痛等领域的主题。研究成果发表于国际期刊Science, Nature, Trends in Neurosciences, Nature Reviews Neuroscience等130多篇。
★
本文使用周围神经损伤模型小鼠,发现小鼠在经历神经损伤后,产生行为性疼痛超敏反应时,会高表达CD11c的脊髓小胶质细胞。CD11c是整合素X(Itgax)家族成员之一,通常在正常组织中,主要表达在髓系细胞;而在神经系统疾病模型中,脑内CD11c小胶质细胞增多。本研究使用Itgax-Venus转基因小鼠(CD11c+细胞表达荧光蛋白Venus),发现清除脊髓CD11c+小胶质细胞,将导致神经损伤小鼠无法从疼痛超敏反应中自发恢复。
此外,研究还发现CD11c+小胶质细胞表达胰岛素样生长因子-1 (IGF1),一旦中断IGF1信号通路,即可导致疼痛超敏性的复发。
综上,本研究揭示了CD11c+小胶质细胞在神经性疼痛的缓解和复发过程中的作用及其机制,为治疗策略提供了潜在的靶点。
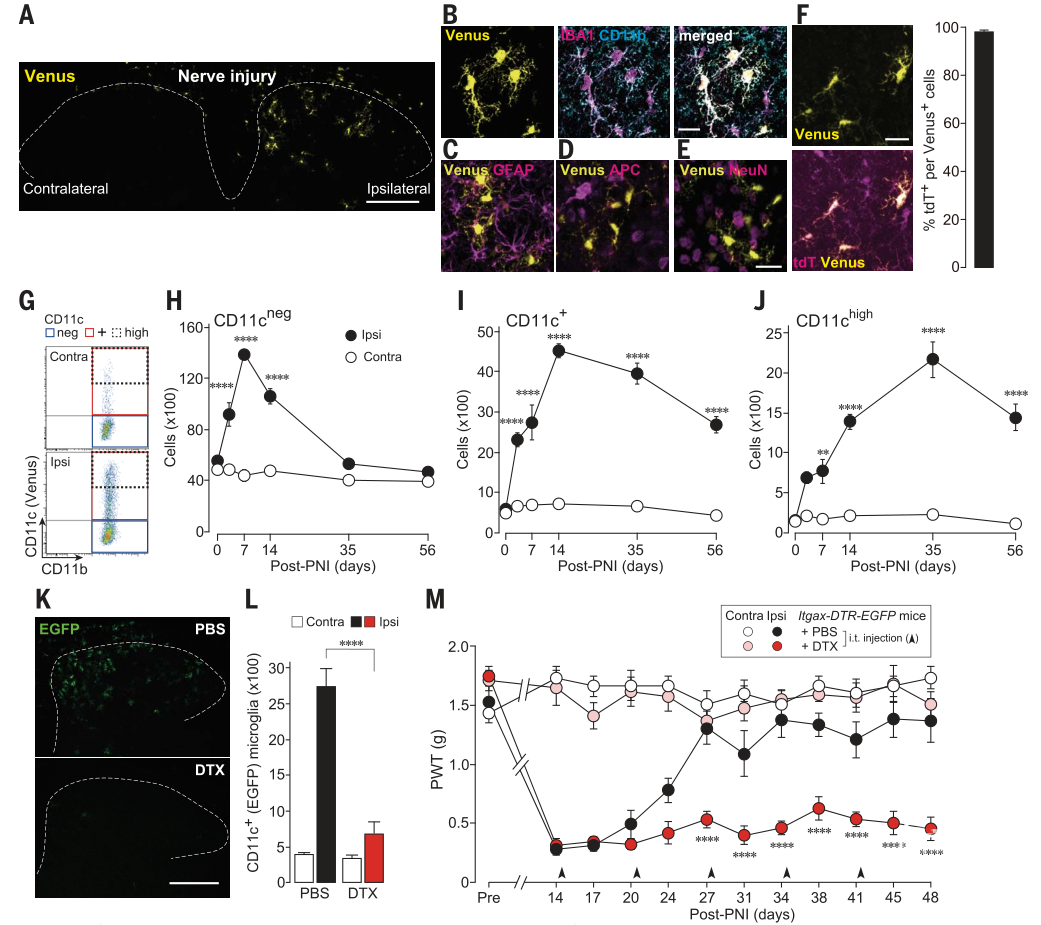
Fig. 1. CD11c+ spinal microglia are necessary for the remission of pain hypersensitivity after PNI. (A) Venus fluorescence in the SDH of Itgax-Venus mice 14 days after PNI. (B to E) Venus and cell-type marker immunostaining: (B) IBA1/CD11b, myeloid markers including microglia; (C) glial fibrillary acidic protein (GFAP), an astrocyte marker; (D) adenomatous polyposis coli (APC), an oligodendrocyte marker; and (E) neuronal nuclei (NeuN), a neuron marker. (F) Venus and tdT fluorescence in the SDH of Hexb tdT/tdT ;Itgax-Venus mice on day 14. Shown is the percentage of tdT+ cells per total Venus+ cells (n = 4 mice). (G toJ) Temporal changes in the number of CD11c neg (H), CD11c+ (I), and CD11c high (J) spinal microglia after PNI (n = 3 to 5 mice) (flow cytometry). (K and L) EGFP in the SDH of Itgax-DTR-EGFP mice 2 days after intrathecal injection of PBS or DTX (0.5 ng) on day 14 (K). Also shown is flow cytometricquantificationofCD11c+ (EGFP) microglia (n = 5 mice) (L). (M) PWT of Itgax-DTR-EGFP mice injected with PBS or DTX before (Pre) and after PNI (n = 7 to 8 mice). Scale bars, 200 mm [(A) and (K)] and 20 mm (B). Data are shown as means ± SEM. **P < 0.01 and ****P < 0.0001.
评语:
本研究强调了CD11c+小胶质细胞对神经性疼痛的缓解和复发重要作用。实验发现小鼠在周围神经损伤数周后能够表现出正常的行为反应,这并非是病理改变的超敏反应的正常化,而是疼痛促进和抑制之间的动态平衡。这种动态平衡依赖于表达IGF-1的CD11c+小胶质细胞的参与。然而,文中使用的周围神经损伤模型中,只观察到脊髓灰质后角的CD11c+小胶质细胞数量的增加;小胶质细胞是否存在区域特异性,文中并没有解释。总而言之,本研究可以为开发治疗神经性疼痛和其他病理症状的治疗策略提供一条有效途径。
关键词:
Neuropathic pain, microglia, CD11c,IGF-1
文章链接DOI:
10.1126/science.abf6805
PMID:
35357926
Abstract
Neuropathic pain is often caused by injury and diseases that affect the somatosensory system. Although pain development has been well studied, pain recovery mechanisms remain largely unknown. Here, we found that CD11c-expressing spinal microglia appear after the development of behavioral pain hypersensitivity following nerve injury. Nerve-injured mice with spinal CD11c+ microglial depletion failed to recover spontaneously from this hypersensitivity. CD11c+ microglia expressed insulin-like growth factor-1 (IGF1), and interference with IGF1 signaling recapitulated the impairment in pain recovery. In pain-recovered mice, the depletion of CD11c+ microglia or the interruption of IGF1 signaling resulted in a relapse in pain hypersensitivity. Our findings reveal a mechanism for the remission and recurrence of neuropathic pain, providing potential targets for therapeutic strategies.
Neuro Oncology
胶质母细胞瘤基因驱动肿瘤相关巨噬细胞/小胶质细胞的功能影响
胶质母细胞瘤(glioblastoma,GBM)是最常见的原发性颅内恶性肿瘤,发病率高,存活率低。GBM会破坏血脑屏障,并且诱导外周骨髓的巨噬细胞浸润,激活常驻小胶质细胞极化。激活的胶质瘤相关的小胶质细胞/巨噬细胞(tumor-associated microglia and macrophages,TAMs)大部分起到促进肿瘤形成和产生免疫抑制的作用。因而TAMs是人类胶质母细胞瘤中重要的微环境成分,是抗肿瘤治疗的潜在靶点。
Q Richard Lu团队于2022年4月1日在国际top期刊《Neuro Oncology》(IF=12.300)发表研究性论文“Glioblastoma Genetic Drivers Dictate the Function of Tumor-Associated Macrophages/Microglia and Responses to CSF1R Inhibition”,论述了胶质瘤生长中功能不同的TAM亚群,并发现间充质样胶质瘤对联合抗血管生成治疗和CSF1R抑制的潜在反应。

Neuro Oncol. (IF 12.300)
★

本研究通讯作者Q Richard Lu,是美国辛辛那提大学教授,是一位发育神经生物学家。他主要研究中枢和外周神经系统的神经肿瘤生物学。实验室主要采用先进的实验方法和开发的新概念和技术的结合,以解决脑肿瘤的发生,复发和治疗的基本问题。近期本实验室使用单细胞转录组学方法来识别发育和胶质瘤形成过程中胶质前体细胞的多样性和细胞命运决定因素,并揭示了髓母细胞瘤中肿瘤发生和复发过程中的肿瘤起始祖细胞和致瘤级联反应。研究成果发表于国际期刊Cell, Cancer Cell, Nature Medicine, Cell Stem Cell, Developmental Cell, Nature Neuroscience等100余篇。
★
本研究构建了同源的PDGFB(血小板生长因子)和ras(RAS基因)驱动的人恶性胶质瘤模型,这种模型类似于前神经样和间充质样胶质瘤,并确定了CSF1R抑制剂PLX3397对胶质瘤生长的影响。此外,作者还探讨TAMs和血管生成对plx3397耐药ras驱动GBM的联合靶向作用。
作者利用单细胞转录组数据分析,考察了PDGFB和ras驱动的胶质瘤中TME细胞组成和功能的差异,确定了不同胶质瘤亚型生长中功能不同的TAM亚群,并发现耐药间充质样胶质瘤对联合抗血管生成治疗和CSF1R抑制的潜在反应。这项研究特别强调了微环境的重要性,为恶性胶质母细胞瘤的靶向治疗提供了新的思路。
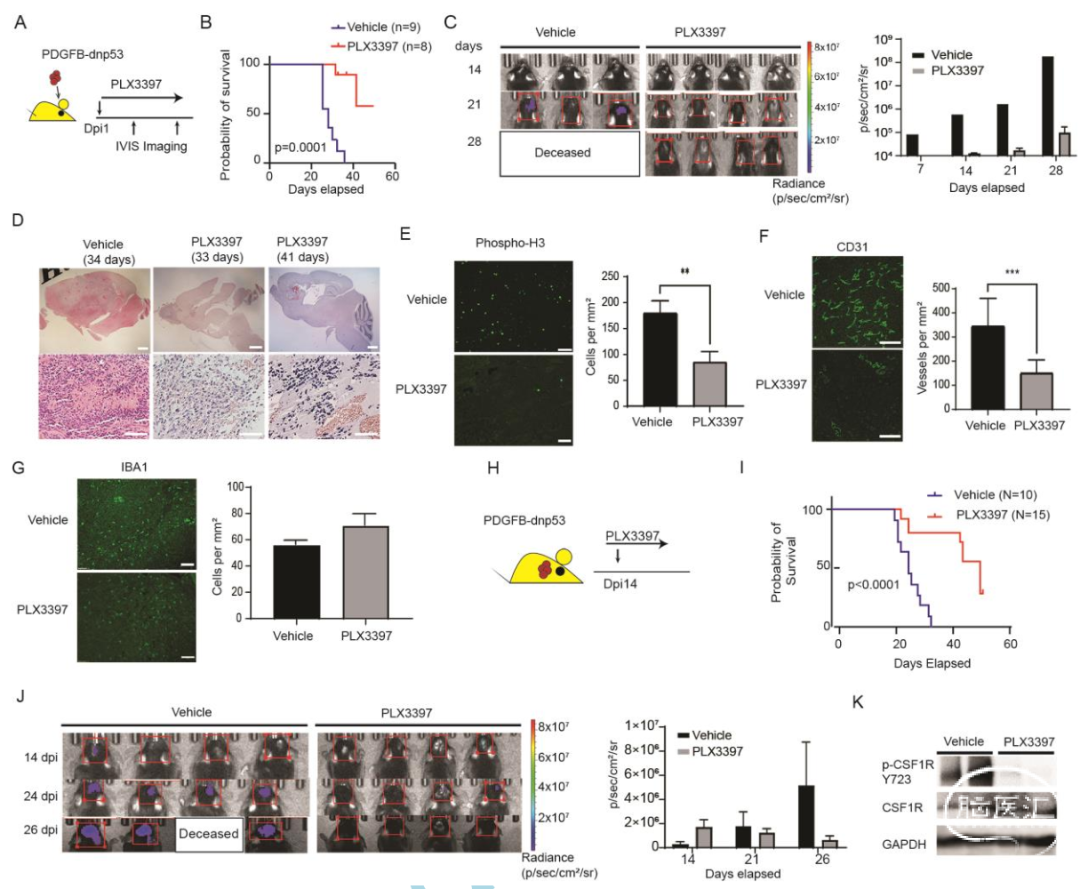
Targeting TAMs blocks tumor growth in murine PDGFB-driven proneural-like gliomas. (A) Experimental design of PLX3397 treatment in PDGFB-driven glioma. Animals were treated every day post-tumor implant (dpi) and monitored by bioluminescent imaging. (B) Kaplan-Meier survival curve of mice treated with PLX3397 or vehicle. Log-rank test. (C) Representative bioluminescent images of mice treated with vehicle or PLX3397. Bar graph: average photon flux at each time point. (D) Representative H&E-stained sections of PDGFB-driven tumors treated with vehicle or PLX3397. Low power scale: 1 mm, High power scale: 300 µM. (E–G) Left: Representative immunostaining for p-H3 (E), CD31 (F), or IBA1 (G) in PDGFB-driven GBM treated with vehicle or PLX3397 for 3 days post-tumor implant. Right: Quantification of label-positive cells per unit area (n = 3 mice/group); **P < .01; ***P < .001, unpaired t test. Scale bars in E and F, 100 µM; G, 300 µM. (H) Experimental design of PLX3397 treatment of established PDGFB-driven tumors. Mice were treated daily with PLX3397 or vehicle beginning 14 days after tumor implantation. (I) Kaplan-Meier survival curve of mice. Log-rank test. (J) Representative bioluminescent images. Bar graph of average photon flux at 14, 21, and 26 days. (K) Western blot of phospho-CSF1R from mouse PDGFB tumors (n = 2 samples per group and repeated twice) treated with vehicle or 100 mg/kg PLX3397 for 2 days from day 14 to 16. Abbreviations: CSF1R, colony-stimulating factor 1 receptor; GBM, glioblastoma; PDGFB, platelet-derived growth factor subunit B; TAMs, tumor-associated macrophages/microglia.
评语:
TAM与胶母细胞瘤的发病机制密切相关,通过促进肿瘤生长、侵袭脑实质从而改变肿瘤微环境,目前的研究表明TAM是一个可行的靶向治疗的靶点,但由于TAM具有多变性,加之中枢神经系统和血脑屏障的复杂性,还需要考虑到患者的耐药性和免疫抑制性,因此从基础研究到临床应用,仍面临着巨大的困难。或许,针对血脑屏障开发一些特殊的靶向药物是未来研究的方向。
关键词:
CSF1R inhibition, angiogenesis, glioblastoma subtypes, single-cell transcriptomics, tumor-associated microglia and macrophages.
文章链接DOI:
10.1093/neuonc/noab228
PMID:
34562087
Abstract
Background: Tumor-associated macrophages/microglia (TAMs) are prominent microenvironment components in human glioblastoma (GBM) that are potential targets for anti-tumor therapy. However, TAM depletion by CSF1R inhibition showed mixed results in clinical trials. We hypothesized that GBM subtype-specific tumor microenvironment (TME) conveys distinct sensitivities to TAM targeting. Methods: We generated syngeneic PDGFB- and RAS-driven GBM models that resemble proneural-like and mesenchymal-like gliomas, and determined the effect of TAM targeting by CSF1R inhibitor PLX3397 on glioma growth. We also investigated the co-targeting of TAMs and angiogenesis on PLX3397-resistant RAS-driven GBM. Using single-cell transcriptomic profiling, we further explored differences in TME cellular compositions and functions in PDGFB- and RAS-driven gliomas. Results: We found that growth of PDGFB-driven tumors was markedly inhibited by PLX3397. In contrast, depletion of TAMs at the early phase accelerated RAS-driven tumor growth and had no effects on other proneural and mesenchymal GBM models. In addition, PLX3397-resistant RAS-driven tumors did not respond to PI3K signaling inhibition. Single-cell transcriptomic profiling revealed that PDGFB-driven gliomas induced expansion and activation of pro-tumor microglia, whereas TAMs in mesenchymal RAS-driven GBM were enriched in pro-inflammatory and angiogenic signaling. Co-targeting of TAMs and angiogenesis decreased cell proliferation and changed the morphology of RAS-driven gliomas. Conclusions: Our work identifies functionally distinct TAM subpopulations in the growth of different glioma subtypes. Notably, we uncover a potential responsiveness of resistant mesenchymal-like gliomas to combined anti-angiogenic therapy and CSF1R inhibition. These data highlight the importance of characterization of the microenvironment landscape in order to optimally stratify patients for TAM-targeted therapy.

声明:脑医汇旗下神外资讯、神介资讯、脑医咨询、AiBrain所发表内容之知识产权为脑医汇及主办方、原作者等相关权利人所有。未经许可,禁止进行转载、摘编、复制、裁切、录制等。经许可授权使用,亦须注明来源。欢迎转发、分享




