
「领域快报」是AiBrain筹备的特别栏目,是由海内外知名高校的一线青年科研工作者(博士后、PI)精选的领域科研动态,旨在为学科融合、交叉合作提供平台和机遇。
「领域快报」神经代谢
PNAS
多巴胺D2受体参与调控肝脏的代谢节律

PNAS (IF 11.205)
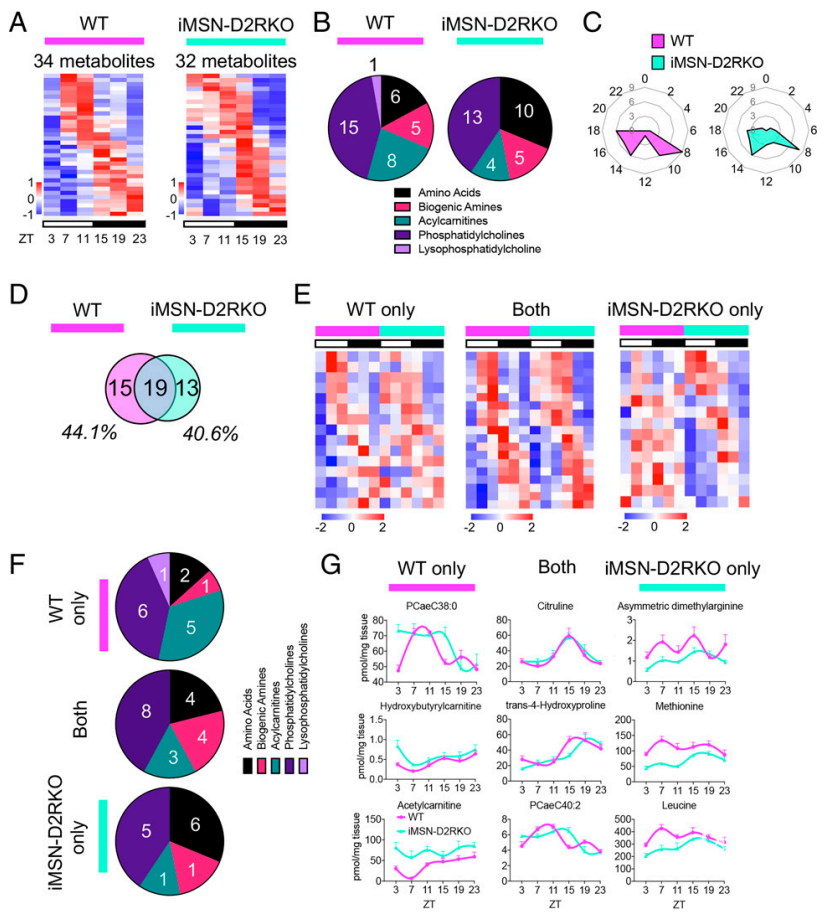
不仅睡眠、觉醒过程存在节律,内脏的代谢也有一定的节律。研究发现多巴胺通路与睡眠觉醒的节律行为高度相关,但是多巴胺信号如何影响外周器官的代谢节律尚不清楚。在本研究中,聚焦于纹状体的MSN神经元中介导间接通路的D2受体。作者敲除小鼠iMSN神经元中的D2受体,发现能够引起小鼠体重降低,摄食减少的代谢相关表型。利用质谱检测肝脏代谢组,发现肝脏代谢的昼夜节律模式也因D2受体缺失而改变。进一步作者以急性给药方式注射可卡因,发现野生型小鼠中枢多巴胺信号响应的同时,肝脏代谢的节律谱也被重新编码,并且这种代谢节律的紊乱在iMSN神经元丢失D2受体的小鼠中更为严重。这些结果揭示了中枢多巴胺通路和肝脏代谢的联系,并从节律的独特视角通过组学分析研究肝脏功能在多巴胺信号变化时受到的影响。
评语:
中医理论中经络、五脏六腑在运行过程中存在昼夜节律,本文通过质谱研究为肝脏代谢节律提供了有力的证据,也为代谢器官的中枢调控机制提供新的视角;同时,本文也扩展了多巴胺系统除经典奖赏功能之外的作用,丰富了领域内对多巴胺系统调控肝脏节律的认知。另外可卡因损伤肝脏代谢节律的表型也提示成瘾患者代谢紊乱的可能机制。本文的重要意义在于引发更多关于代谢器官节律和功能神经机制的思考,未来可以对肝脏代谢节律的神经环路机制、分子机制展开更为细致的研究。
关键词:
medium spiny neurons (MSNs);dopamine D2 receptor;liver;metabolomic
文章链接 :
https://doi.org/10.1073/pnas.2117113119
Abstract
The circadian clock is tightly intertwined with metabolism and relies heavily on multifaceted interactions between organ systems to maintain proper timing. Genetic and/or environmental causes can disrupt communication between organs and alter rhythmic activities. Substance use leads to altered dopamine signaling followed by reprogramming of circadian gene expression and metabolism in the reward system. However, whether altered dopamine signaling in the brain affects circadian metabolism in peripheral organs has not been fully explored. We show that dopamine D2 receptors (D2R) in striatal medium spiny neurons (MSNs) play a key role in regulating diurnal liver metabolic activities. In addition, drugs that increase dopamine levels, such as cocaine, disrupt circadian metabolic profiles in the liver, which is exacerbated by loss of D2R signaling in MSNs. These results uncover a strict communication between neurons/brain areas and liver metabolism as well as the association between substance use and systemic deficits.
Nature biomedical engineering
超声刺激肝神经丛由TRPA1通道介导影响PVN神经活动改善代谢表型

Nature biomedical engineering (IF 25.671)
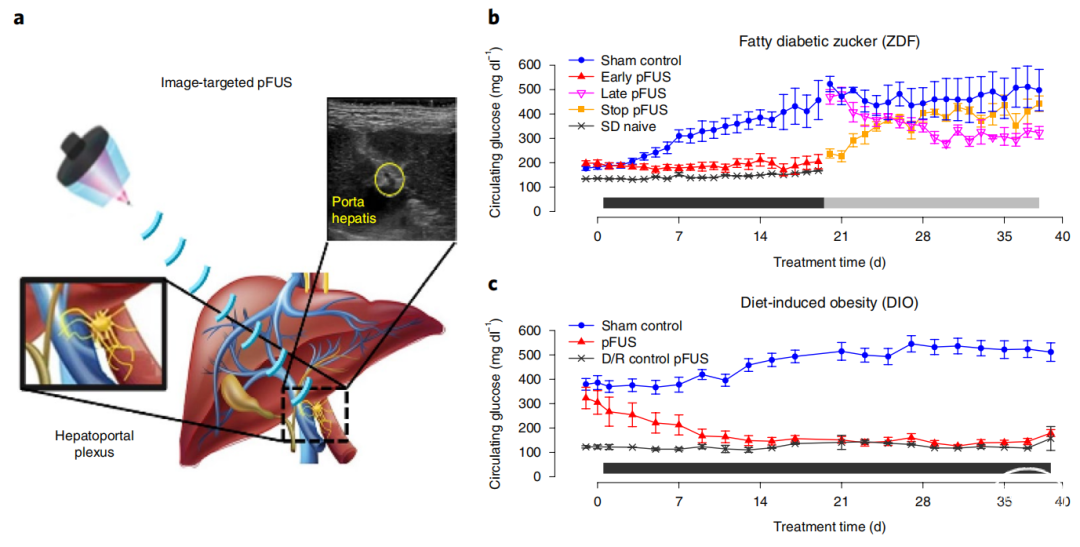
大脑对代谢平衡的调控依赖于外周神经元对营养物质的感知以及自主神经系统对代谢器官功能的调节。本研究关注糖代谢的主要器官——肝脏,结果表明通过外周聚焦超声刺激peripheral focused ultrasound stimulation(pFUS)干预肝脏门静脉神经丛,能够降低2型糖尿病动物模型的高血糖。
每天3min的重复慢性刺激可以促进葡萄糖摄取和糖原累积,并改善葡萄糖耐量和胰岛素敏感性,并且这一作用与下丘脑神经肽Y(NPY)的下调相关。转录组分析表明,外周超声刺激也改变了代谢器官组织中的基因表达。利用药物或手术去神经支配后,超声刺激的作用消失,表明这一作用由肝脏和大脑之间的自主神经系统所介导。进一步研究发现响应超声刺激的TRPA1离子通道是超声刺激起效的必要条件。
综上所述,本文发展出激活自主神经系统的无创超声刺激方式,缓解糖尿病等代谢疾病中葡萄糖稳态的失调,并探究其机制,为临床治疗代谢疾病提供新的神经干预策略。
评语:
除了药物治疗和手术治疗外,通过生物医学工程或各种物理手段实现的神经调控策略已经被广泛用于诸多疾病的临床治疗,甚至包括免疫疾病和代谢疾病等传统上被认为是非神经系统的疾病,如类风湿关节炎和肠易激综合征等。但运用如经皮磁刺激、经颅磁刺激以及深部脑刺激等物理手段治疗疾病,存在一定的局限性,如刺激深度不够,不能够准确到达目标靶点,或者需要有创植入设备,有一定风险。本文提供的外周神经超声刺激方式无创安全,也可以避免药物的副作用。
关键词:
peripheral focused ultrasound stimulation(pFUS);Glucose homoeostasis;hepathoportal nerve plexus;PVN;NPY
文章链接 :
https://www.nature.com/articles/s41551-022-00870-w
Abstract
Peripheral neurons that sense glucose relay signals of glucose availability to integrative clusters of neurons in the brain. However, the roles of such signalling pathways in the maintenance of glucose homoeostasis and their contribution to disease are unknown. Here we show that the selective activation of the nerve plexus of the hepatic portal system via peripheral focused ultrasound stimulation (pFUS) improves glucose homoeostasis in mice and rats with insulin-resistant diabetes and in swine subject to hyperinsulinemic-euglycaemic clamps. pFUS modulated the activity of sensory projections to the hypothalamus, altered the concentrations of metabolism-regulating neurotransmitters, and enhanced glucose tolerance and utilization in the three species, whereas physical transection or chemical blocking of the liver–brain nerve pathway abolished the effect of pFUS on glucose tolerance. Longitudinal multi-omic profiling of metabolic tissues from the treated animals confirmed pFUS-induced modifications of key metabolic functions in liver, pancreas, muscle, adipose, kidney and intestinal tissues. Non-invasive ultrasound activation of afferent autonomic nerves may represent a non-pharmacologic therapy for the restoration of glucose homoeostasis in type-2 diabetes and other metabolic diseases.
Cell Metabolism
PVN催产素神经元通过交感神经支配胰岛β细胞分泌胰岛素从而调节血糖

Cell metabolism (IF 27.287)
中枢在调控血糖过高和胰岛素分泌中起重要作用,这个共识在代谢领域已存在百年以上。以往的研究通过病毒示踪解析了中枢与胰腺之间的神经投射,然而大脑和胰岛β细胞之间的解剖和功能连接还需要进一步阐明。
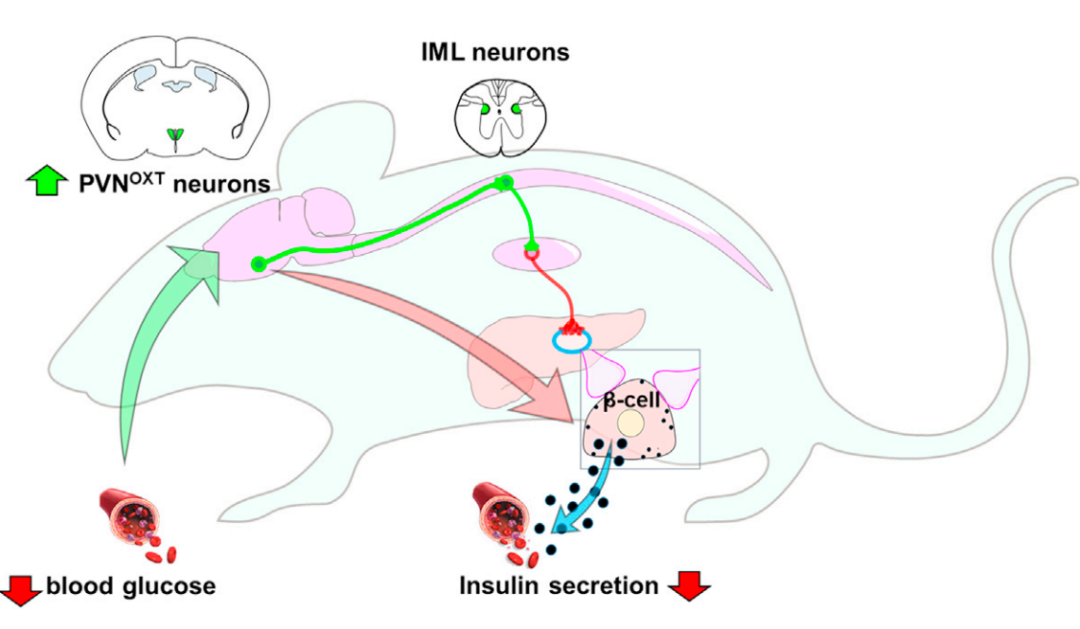
在这篇文章中,作者利用伪狂犬病毒追踪下丘脑室旁核(paraventricular hypothalamic nucleus,PVN)的OXT神经元(oxytocin neurons,催产素神经元)通过交感神经向胰腺发出功能投射。通过光遗传学,发现激活PVNOXT神经元会迅速抑制胰岛β细胞分泌胰岛素,并造成血糖升高;而光遗传学抑制则产生相反的效果。进一步研究发现PVNOXT的活性会由于葡萄糖缺乏而被激活。综上所述,本文揭示了通过多级投射调控胰岛β细胞分泌胰岛素的中枢脑区,拓展了我们对外周器官与中枢神经相互作用及及其机制的认知。
评语:
领域内很早就发现自主神经系统参与代谢器官功能的调控,但是对其上游中枢的脑区机制和细胞类型机制并不明确。本研究揭示了摄食和代谢关键脑区PVN通过多级神经投射,调控胰腺释放胰岛素的功能,并解析出其精细的神经元类型机制。此外既往研究表明PVN通过整合下丘脑其他亚区的信号,向肝脏发出多级神经投射,调控其代谢功能,本研究丰富了我们对于PVN与除肝脏外的代谢器官之间功能投射的认识。
关键词:
paraventricular hypothalamic nucleus(PVN);oxytocin neurons(OXT);pancreatic b cells;insulin secretion
文章链接:
https://doi.org/10.1016/j.cmet.2021.12.020
Abstract
The central nervous system has long been thought to regulate insulin secretion, an essential process in the maintenance of blood glucose levels. However, the anatomical and functional connections between the brain and insulin-producing pancreatic β cells remain undefined. Here, we describe a functional transneuronal circuit connecting the hypothalamus to β cells in mice. This circuit originates from a subpopulation of oxytocin neurons in the paraventricular hypothalamic nucleus (PVNOXT), and it reaches the islets of the endocrine pancreas via the sympathetic autonomic branch to innervate β cells. Stimulation of PVNOXT neurons rapidly suppresses insulin secretion and causes hyperglycemia. Conversely, silencing of these neurons elevates insulin levels by dysregulating neuronal signaling and secretory pathways in β cells and induces hypoglycemia. PVNOXT neuronal activity is triggered by glucoprivation. Our findings reveal that a subset of PVNOXT neurons form functional multisynaptic circuits with β cells in mice to regulate insulin secretion, and their function is necessary for the β cell response to hypoglycemia.

声明:脑医汇旗下神外资讯、神介资讯、脑医咨询、AiBrain所发表内容之知识产权为脑医汇及主办方、原作者等相关权利人所有。未经许可,禁止进行转载、摘编、复制、裁切、录制等。经许可授权使用,亦须注明来源。欢迎转发、分享




