「领域快报」是AiBrain筹备的特别栏目,是由海内外知名高校的一线青年科研工作者(博士后、PI)精选的领域科研动态,旨在为学科融合、交叉合作提供平台和机遇。
「领域快报」神经炎症及免疫
Nature
肺部肠道微生物调控大脑自免疫功能

Nature (IF 49.962)
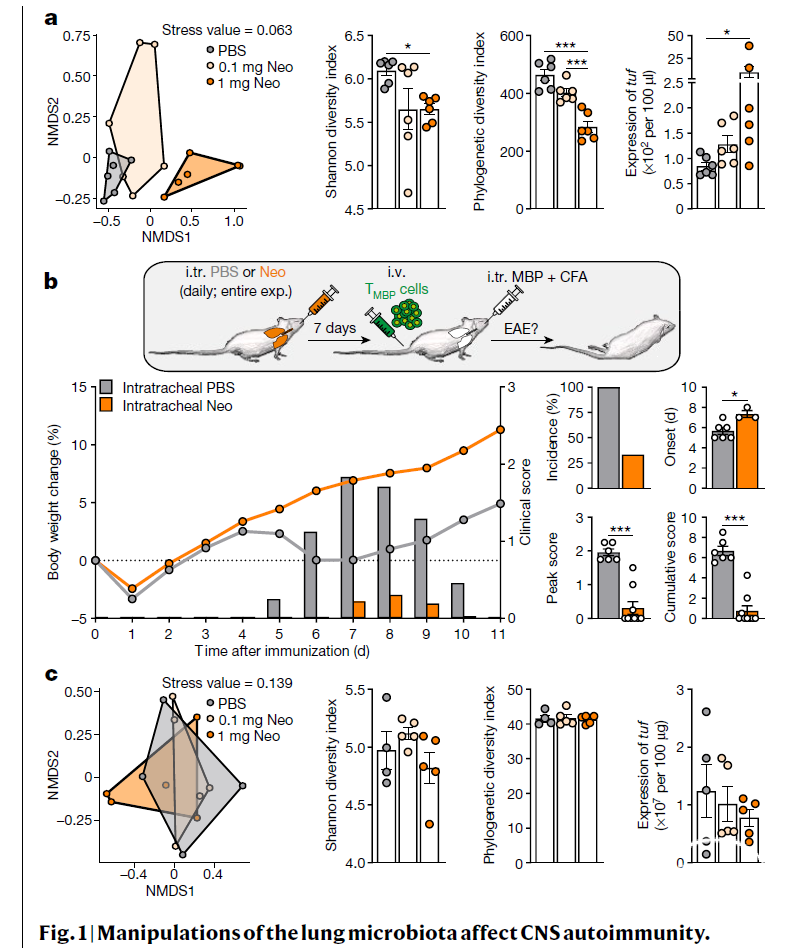
肺部感染和吸烟是多发性硬化症(multiple sclerosis)的风险因子。多发性硬化症是一种T细胞介导的中枢神经系统自体免疫疾病。此外,肺部可以作为一个适宜的小环境利于诱导疾病的T细胞长期存活以及成熟为具有迁移能力的效应T细胞。但是为什么肺组织对大脑自体免疫疾病有如此重要的作用尚不清楚。本文检测到了肺部微生物组与大脑免疫反应之间紧密的相互联系。肺部微生物组的失调显著影响了大鼠对中枢神经系统自体免疫疾病的易感性。通过新霉素(neomycin)局部处理将肺部微生物组调节成富含脂多糖的微生物门类(phyla)能够诱导大脑驻留的小胶质细胞的I型干扰素引发状态。他们对II型干扰素以自体免疫为主的刺激的反应受损,导致促炎症反应、免疫细胞募集和临床症状减少。使用多粘菌素B(polymyxin B)抑制产脂多糖的肺部微生物门类,导致疾病加剧,而添加富含脂多糖的微生物门类或脂多糖能够重复出新霉素处理产生的效果。总之,本文证明了肺脑轴的存在,以及肺微生物组调节中枢神经组织的免疫活性,从而影响其对自体免疫疾病发展的易感性。
评语:
本文提出了肺脑轴的概念,温和的肺部微生物组改变能够通过小胶质细胞影响大脑的自体免疫活性。肺部微生物组又容易受到肺部感染、吸烟、药物治疗以及环境因素影响,通过肺脑轴作用推测,这些因素可能会影响大脑的免疫活性,进而影响大脑功能。另一方面,从临床的角度来看,或许可以通过调节肺部微生物组来改善大脑免疫活性变化相关联的疾病。该概念的提出会启发更多关于肺脑轴的研究。
关键词:
Lung microbiome, brain autoimmunity, microglia, lipopolysaccharide, lung-brain axis
PMID :
35197636
文章链接 :
https://www.nature.com/articles/s41586-022-04427-4
Abstract
Lung infections and smoking are risk factors for multiple sclerosis, a T-cell-mediated autoimmune disease of the central nervous system1. In addition, the lung serves as a niche for the disease-inducing T cells for long-term survival and for maturation into migration-competent effector T cells2. Why the lung tissue in particular has such an important role in an autoimmune disease of the brain is not yet known. Here we detected a tight interconnection between the lung microbiota and the immune reactivity of the brain. A dysregulation in the lung microbiome significantly influenced the susceptibility of rats to developing autoimmune disease of the central nervous system. Shifting the microbiota towards lipopolysaccharide-enriched phyla by local treatment with neomycin induced a type-I-interferon-primed state in brain-resident microglial cells. Their responsiveness towards autoimmune-dominated stimulation by type II interferons was impaired, which led to decreased proinflammatory response, immune cell recruitment and clinical signs. Suppressing lipopolysaccharide-producing lung phyla with polymyxin B led to disease aggravation, whereas addition of lipopolysaccharide-enriched phyla or lipopolysaccharide recapitulated the neomycin effect. Our data demonstrate the existence of a lung-brain axis in which the pulmonary microbiome regulates the immune reactivity of the central nervous tissue and thereby influences its susceptibility to autoimmune disease development.
Nature neuroscience
肠道微生物组通过代谢物驱使小胶质细胞衰老相关的氧化应激和线粒体损伤
Nature Neuroscience(IF 24.884)
小胶质细胞功能随着衰老而减退。小胶质细胞与肠道微生物组的相互作用在机体发育和成年阶段已经得到很好的研究,但在衰老过程的研究较少。本文比较了无菌和无特定病原菌条件下饲养的年轻成年小鼠和老年小鼠的小胶质细胞转录组,发现微生物组影响了与衰老相关的小胶质细胞基因表达的变化。肠道微生物组的缺失减少了老年小鼠大脑小胶质细胞的氧化应激水平并减轻了线粒体功能损伤。对血清和脑组织的无差别的宏代谢组学分析显示N6-羧甲基赖氨酸(CML)在衰老大脑的小胶质细胞中累积。CML介导了活性氧的激增,并且抑制了小胶质细胞线粒体活性和ATP存储。本文也在人脑和血清中验证了CML水平的年龄依赖性升高。最后,本文发现老年小鼠中微生物组依赖的肠道通透性的提高介导了CML水平的升高。这项研究为肠道微生物组如何调节小胶质细胞特定特征提供了更多思路。
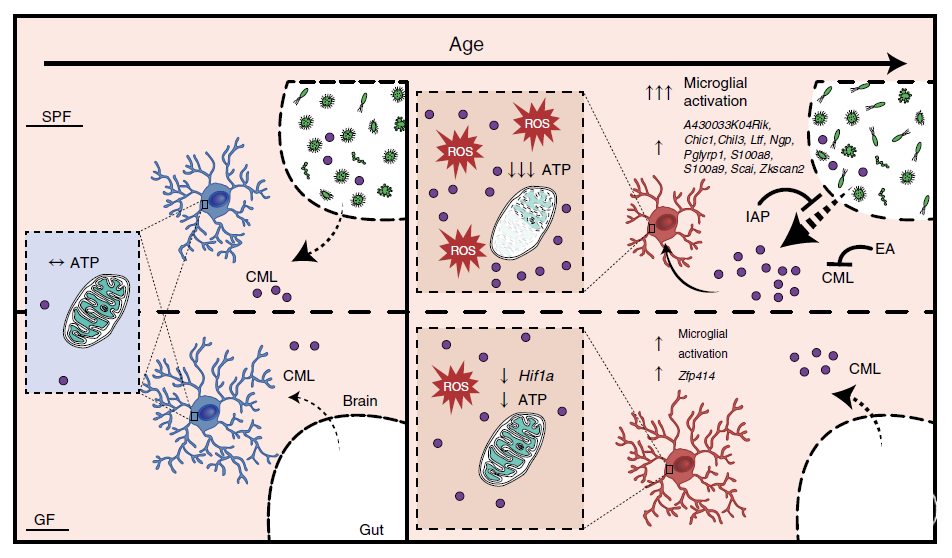
Schematic of gut microbiota-mediated CML accumulation in the aged brain. CML induces aging features in microglia, specifically oxidative stress and mitochondrial damage. Rejuvenating the gut–blood barrier integrity limits CML accumulation and its detrimental effects on microglia.
评语:
肠道菌群通过小胶质细胞影响大脑免疫功能的研究较多,本文从一个全新的角度,即生物体衰老阶段肠道菌群是如何影响大脑功能的。本文发现菌群代谢物N6-羧甲基赖氨酸(CML)能够通过突然增加小胶质细胞活性氧水平,抑制线粒体活性来改变大脑的免疫功能,这也为菌群代谢物影响大脑健康提供了更多有力的证据。
关键词:
aging, microglia, gut microbiota, N6-carboxymethyllysine (CML)
PMID :
35241804
文章链接 :
https://www.nature.com/articles/s41593-022-01027-3
Abstract
Microglial function declines during aging. The interaction of microglia with the gut microbiota has been well characterized during development and adulthood but not in aging. Here, we compared microglial transcriptomes from young-adult and aged mice housed under germ-free and specific pathogen-free conditions and found that the microbiota influenced aging associated-changes in microglial gene expression. The absence of gut microbiota diminished oxidative stress and ameliorated mitochondrial dysfunction in microglia from the brains of aged mice. Unbiased metabolomic analyses of serum and brain tissue revealed the accumulation of N6-carboxymethyllysine (CML) in the microglia of the aging brain. CML mediated a burst of reactive oxygen species and impeded mitochondrial activity and ATP reservoirs in microglia. We validated the age-dependent rise in CML levels in the sera and brains of humans. Finally, a microbiota-dependent increase in intestinal permeability in aged mice mediated the elevated levels of CML. This study adds insight into how specific features of microglia from aged mice are regulated by the gut microbiota.
Molecular Neurodegeneration
AD中肠道微生物组对神经炎症和突触异常功能的影响

Molecular Neurodegeneration (IF 14.195)
背景:
应用肠道菌群在健康和疾病中控制大脑功能是一个新的、目前正在出现的概念。越来越多的研究数据提示,肠道菌群至少部分通过调节神经炎症发挥作用。鉴于神经炎症变化和神经元活动之间的联系,肠道菌群可能通过影响神经炎症的关键参与者小胶质细胞来间接影响神经元功能是合理的。事实上,日益增长的证据显示菌群等因素可能参与到小胶质细胞和突触功能失调之间的相互作用。除了菌群对神经元活动的这些间接的小胶质细胞依赖性作用外,最近的研究表明菌群还可以通过直接刺激迷走神经来影响神经元活动。
主要内容:
通过关注AD,本文讨论了菌群直接或者间接影响神经元活动的可能机制。AD是研究最多的神经退行性疾病之一,也是全球痴呆主要类型。更具体地说,在外周和中枢炎症交互作用的背景下讨论了菌群介导的小胶质细胞改变的机制。后续,本文突出了菌群在调节外周免疫体液介质中的作用,及其对迷走神经的刺激影响。
最后,本文探讨了对于菌群的干扰是否能够影响,以及如何影响突触传递和下游的认知功能障碍。
结论:
越来越多的有力证据支持肠道菌群在AD病理中的作用,包括对导致认知功能衰退的突触功能障碍和神经炎症的影响。基于菌群调节的早期干预策略可能对AD有治疗前景,但仍需进一步研究。
评语:
本文从肠道菌群影响神经炎症的角度,系统总结了肠道菌群影响AD发生的病理机制和可能的治疗思路。
关键词:
Alzheimer’s disease; Gut microbiota; Neuroinflammation; Peripheral immunomodulation; Synaptic dysfunction.
PMID :
35248147
文章链接 :
https://molecularneurodegeneration.biomedcentral.com/articles/10.1186/s13024-022-00522-2
Abstract
Background: The implication of gut microbiota in the control of brain functions in health and disease is a novel, currently emerging concept. Accumulating data suggest that the gut microbiota exert its action at least in part by modulating neuroinflammation. Given the link between neuroinflammatory changes and neuronal activity, it is plausible that gut microbiota may affect neuronal functions indirectly by impacting microglia, a key player in neuroinflammation. Indeed, increasing evidence suggests that interplay between microglia and synaptic dysfunction may involve microbiota, among other factors. In addition to these indirect microglia-dependent actions of microbiota on neuronal activity, it has been recently recognized that microbiota could also affect neuronal activity directly by stimulation of the vagus nerve.
Main messages: The putative mechanisms of the indirect and direct impact of microbiota on neuronal activity are discussed by focusing on Alzheimer's disease, one of the most studied neurodegenerative disorders and the prime cause of dementia worldwide. More specifically, the mechanisms of microbiota-mediated microglial alterations are discussed in the context of the peripheral and central inflammation cross-talk. Next, we highlight the role of microbiota in the regulation of humoral mediators of peripheral immunity and their impact on vagus nerve stimulation. Finally, we address whether and how microbiota perturbations could affect synaptic neurotransmission and downstream cognitive dysfunction.
Conclusions: There is strong increasing evidence supporting a role for the gut microbiome in the pathogenesis of Alzheimer's disease, including effects on synaptic dysfunction and neuroinflammation, which contribute to cognitive decline. Putative early intervention strategies based on microbiota modulation appear therapeutically promising for Alzheimer's disease but still require further investigation.

声明:脑医汇旗下神外资讯、神介资讯、脑医咨询、AiBrain所发表内容之知识产权为脑医汇及主办方、原作者等相关权利人所有。未经许可,禁止进行转载、摘编、复制、裁切、录制等。经许可授权使用,亦须注明来源。欢迎转发、分享




