「领域快报」是AiBrain筹备的特别栏目,是由海内外知名高校的一线青年科研工作者(博士后、PI)精选的领域科研动态,旨在为学科融合、交叉合作提供平台和机遇。
「领域快报」神经退行性疾病
Nat Rev Drug Discov
基于淀粉样多肽假说治疗AD的新观点

Nat Rev Drug Discov (IF 84.694)
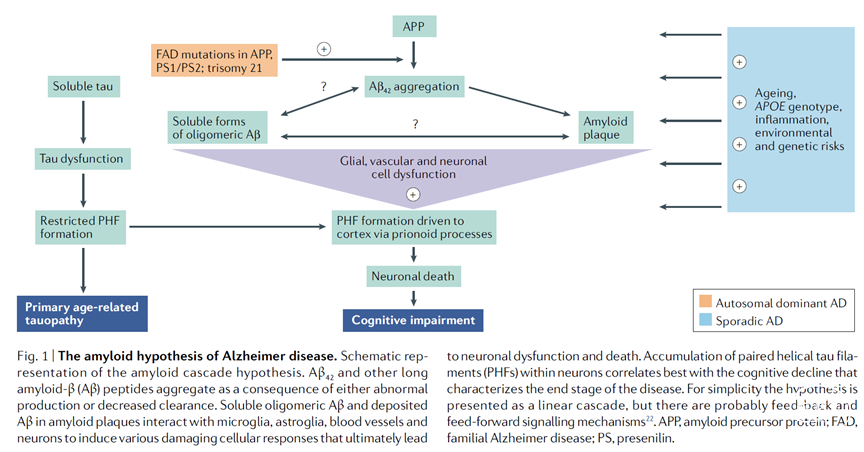
多个靶向β淀粉样多肽(Aβ)的AD药物在临床试验中被证明无效。即便如此,4个针对β淀粉样多肽的抗体药物显示能够介导清除AD病人大脑β淀粉样斑。最近,FDA通过使用淀粉样斑水平降低作为替代评价终点,加速审批通过了其中一个单抗药物,即阿杜卡单抗(aducanumab)。目前抗体药物被审批通过的合理性和其临床效果仍有巨大争议。本文提出了这个争议,从AD的两种病态蛋白聚合物的时间交互作用-β淀粉样斑和tau神经纤维缠结-以及它们之间的关系对认知功能损伤的角度,综述了针对靶向Aβ药物的临床试验数据,突出了能够影响临床疗效的药物特征的差异性。基于此,作者提出Aβ病理症状驱动了tao蛋白病理症状,β淀粉样斑需要被降低到一定水平(~20 centiloids)才能显示出显著的临床益处,并且Aβ的清除与临床效果之间有一定的延迟。作者总结,在临床实验中,大脑中淀粉样多肽被潜在治疗药物清除的速度是证明其临床获益的关键因素。
评语:
针对降低AD患者Aβ水平的大约20种药物在临床试验中均以失败告终,而aducanumab作为单抗药物降低了在AD脑内降低Aβ,FDA以此为评价终点加速审批通过它的使用,即使争议巨大,但对AD药物开发领域注入了信心。
关键词:
amyloid-β (Aβ); Alzheimer disease (AD); aducanumab
文章链接 :
https://www.nature.com/articles/s41573-022-00391-w
Abstract
Many drugs that target amyloid-β (Aβ) in Alzheimer disease (AD) have failed to demonstrate clinical efficacy. However, four anti-Aβ antibodies have been shown to mediate the removal of amyloid plaque from brains of patients with AD, and the FDA has recently granted accelerated approval to one of these, aducanumab, using reduction of amyloid plaque as a surrogate end point. The rationale for approval and the extent of the clinical benefit from these antibodies are under intense debate. With the aim of informing this debate, we review clinical trial data for drugs that target Aβ from the perspective of the temporal interplay between the two pathognomonic protein aggregates in AD - Aβ plaques and tau neurofibrillary tangles - and their relationship to cognitive impairment, highlighting differences in drug properties that could affect their clinical performance. On this basis, we propose that Aβ pathology drives tau pathology, that amyloid plaque would need to be reduced to a low level (~20 centiloids) to reveal significant clinical benefit and that there will be a lag between the removal of amyloid and the potential to observe a clinical benefit. We conclude that the speed of amyloid removal from the brain by a potential therapy will be important in demonstrating clinical benefit in the context of a clinical trial.
Neurology
阿杜卡单抗(aducanumab)治疗AD的临床证据

Neurology (IF 9.910)
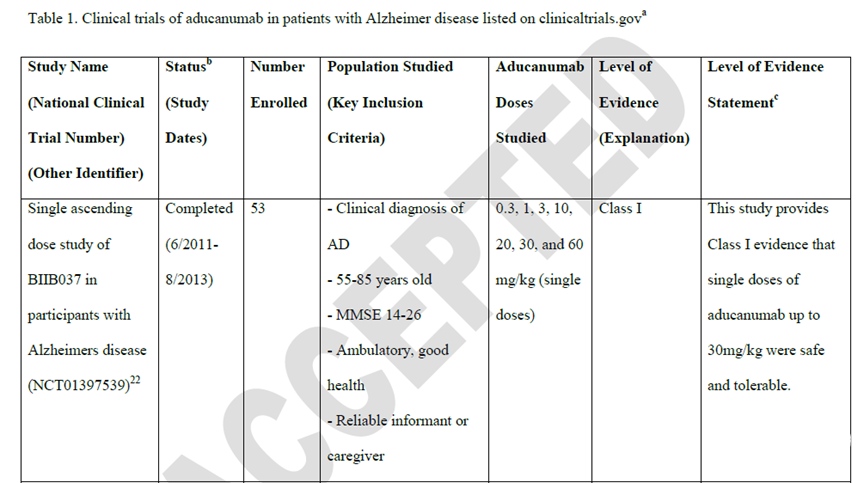
本文目标是提供关于阿杜卡单抗(aducanumab)治疗AD的临床证据,为临床使用该药物做参考。作者系统性综述了现有的关于aducanumab在早期症状性AD患者服用过程中的详细的临床数据。各级证据表述依据2017年美国神经学会的基于证据方案的治疗分类来进行划分。同时,文章也综述了安全信息,监管决定以及临床背景等信息。本文获取了4项(1项I类和3项II类)临床试验的数据。I类研究显示高达30 mg/kg的单剂量aducanumab是安全的且有较好的耐受性。三项II类研究证据显示,与安慰剂组相比,3-10 mg/kg剂量的aducanumab治疗一年后,PET结果显示aducanumab能够降低淀粉样多肽在脑内的沉积。II类研究的有效性数据并不一致,随着剂量和结果的变化有所差异。aducanumab要么在临床痴呆评定量表总和评分上对平均变化没有影响,要么导致更少的病情加剧作用(与安慰剂相比)。可逆的的淀粉样多肽相关的图像异常在大约40%的aducanumab治疗个体中发生,而只有10%的安慰剂组个体出现图像异常。本文也提供了几点aducanumab临床使用相关的总结。aducanumab给药将需要扩大的临床基础设施。需要基于证据的指导来处理关键问题,(例如研究群体的安全性没有包括到III期研究中,病人日常功能的预期获益,治疗周期)以及对于获取aducanumab相关的关键问题(例如覆盖率、花费、月度输液负担),这些问题将会帮助病人和药物提供方共同作出治疗决定。
注:此处仅展示部分图标
关键词:
amyloid-β (Aβ); Alzheimer disease (AD); aducanumab; clinical trials
PMID:
35197360 DOI: 10.1212/WNL.0000000000200176
文章链接 :
https://n.neurology.org/content/early/2022/02/23/WNL.0000000000200176
Abstract
Objective: To identify the class of evidence for aducanumab use for the treatment of Alzheimer disease and present clinical considerations regarding use.
Methods: The author panel systematically reviewed available clinical trial data detailing aducanumab use in individuals with early symptomatic Alzheimer disease. Level of evidence statements were assigned in accordance with the American Academy of Neurology's 2017 therapeutic classification of evidence scheme. Safety information, regulatory decisions, and clinical context were also reviewed.
Results: Data were identified from 4 clinical trials, 1 rated Class I and 3 rated Class II. The Class I study showed that single doses of aducanumab up to 30 mg/kg were safe and well tolerated. All 3 Class II studies provided evidence that aducanumab (3-10 mg/kg) decreased amyloid deposition on brain PET at 1-year vs placebo. Efficacy data in the Class II studies varied by dose and outcome, but aducanumab either had no effect on mean change on the Clinical Dementia Rating® Sum-of-Boxes scores or resulted in less worsening (vs placebo) that was of uncertain clinical importance. Adverse amyloid-related imaging abnormalities occurred in approximately 40% of individuals treated with aducanumab vs 10% receiving placebo.
Clinical context: Administration of aducanumab will require expanded clinical infrastructure. Evidence-based guidance is needed to address key questions (e.g., safety in populations not enrolled in Phase 3 studies, expected benefits on daily function, treatment duration) and critical issues relating to access to aducanumab (e.g., coverage, costs, burden of monthly infusions) that will inform shared decision-making between patients and providers.
Aging Cell
伽马频率的光闪烁改善AD病理症状的作用机制

Aging Cell (IF 9.304)
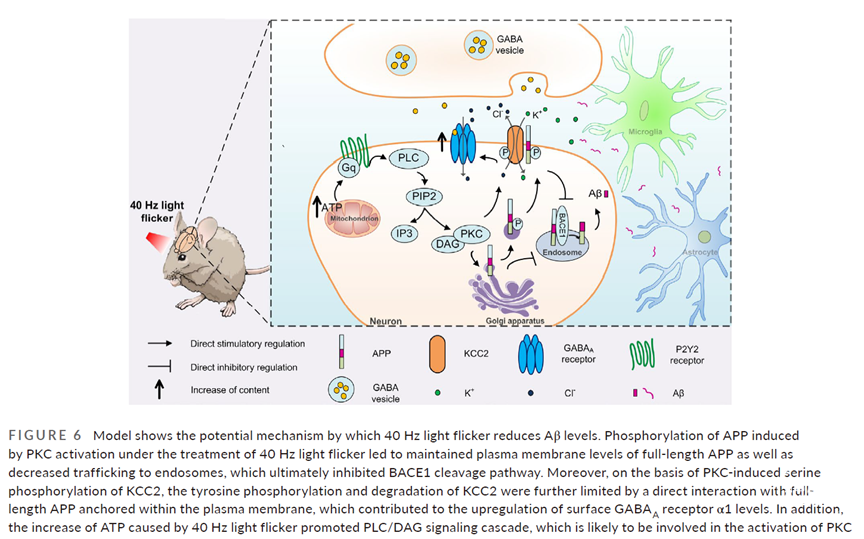
非侵入式光闪烁诱导的伽马振荡已被报告能够影响AD相关的病理症状。但是,现在仍然不清楚哪些信号通路参与其中来下调淀粉样多肽水平。本文发现,伽马频率的光闪烁能够促进淀粉样多肽前体蛋白(APP,amyloid precursor protein)锚定在细胞质膜上,进行非淀粉样多肽生成过程( non-amyloidogenic processing)。然后APP与KCC2(一种神经元特定的钾离子氯离子共转运蛋白)相互作用,提示APP与KCC2的相互作用对于维持表面GABAA受体的α1水平以及降低β淀粉样多肽的生成是必要的。这种光闪烁刺激能够限制KCC2的内化以及后续的通过酪氨酸磷酸化和泛素化的降解,导致细胞表面KCC2水平的上升。更加特别的是,伽马频率的光闪烁能够引起PKC依赖的APP丝氨酸残基的磷酸化,这对维持细胞质膜全长APP的水平起主要作用,并最终降低APP被转运到胞内体进而抑制了β剪切酶通路。伽马频率的光闪烁激活的PKC随后能够磷酸化KCC2的丝氨酸,并将KCC2稳定在细胞表面,帮助上调了细胞表面GABAA受体α1的水平。总之,本文的研究提示在AD中通过广闪烁来增强APP转运至细胞质膜对下调Aβ水平起到重要的调节作用。
关键词:
Alzheimer's disease; GABAA receptor α1; KCC2; amyloid precursor protein trafficking; gamma frequency light flicker; β-amyloid.
PMID:
35199454 DOI: 10.1111/acel.13573
文章链接 :
https://onlinelibrary.wiley.com/doi/10.1111/acel.13573
Abstract
Inducing gamma oscillations with non-invasive light flicker has been reported to impact Alzheimer's disease-related pathology. However, it is unclear which signaling pathways are involved in reducing amyloid load. Here, we found that gamma frequency light flicker increased anchoring of amyloid precursor protein (APP) to the plasma membrane for non-amyloidogenic processing, and then physically interacted with KCC2, a neuron-specific K+ -Cl- cotransporter, suggesting that it is essential to maintain surface GABAA receptor α1 levels and reduce β-amyloid (Aβ) production. Stimulation with such light flicker limited KCC2 internalization and subsequent degradation via both tyrosine phosphorylation and ubiquitination, leading to an increase in surface-KCC2 levels. Specifically, PKC-dependent phosphorylation of APP on a serine residue was induced by gamma frequency light flicker, which was responsible for maintaining plasma membrane levels of full-length APP, leading to its reduced trafficking to endosomes and inhibiting the β-secretase cleavage pathway. The activated PKC from the gamma frequency light flicker subsequently phosphorylated serine of KCC2 and stabilized it onto the cell surface, which contributed to the upregulation of surface GABAA receptor α1 levels. Together, these data indicate that enhancement of APP trafficking to the plasma membrane via light flicker plays a critical modulatory role in reduction of Aβ load in Alzheimer's disease.

声明:脑医汇旗下神外资讯、神介资讯、脑医咨询、AiBrain所发表内容之知识产权为脑医汇及主办方、原作者等相关权利人所有。未经许可,禁止进行转载、摘编、复制、裁切、录制等。经许可授权使用,亦须注明来源。欢迎转发、分享。




