今天为大家分享的是由王小峰医师编译,陈成伟医师审校的:经外侧裂选择性海马-杏仁核切除手术策略与技巧,欢迎观看、阅读。
Transsylvian selective amygdalohippocampectomy
Selective amygdalohippocampectomy 1
Selective amygdalohippocampectomy 2
Anteromedial Temporal Lobectomy and Selective Amygdalohippocampectomy
概述
药物难治性颞叶内侧癫痫已有多种外科治疗方案,效果均良好。经外侧裂选择性海马-杏仁核切除术(SA)是最初用于最大限度的保留颞叶皮质完整的手术技术,也是其中最考验手术技巧一项外科方案,与标准颞叶前内侧、海马-杏仁核切除术(ATL)(包含切除部分颞叶外侧皮质)相比,最近一些研究表明SA术后抗癫痫效果相似,神经心理预后更好,但此类研究争议较大,部分病人的治疗效果并不确切。《颞叶前内侧切除术》将在专门章节中讨论。
SA与ALT相比,手术操作复杂度更高、不可预测风险更多,需要熟练的术中神经显微技术,因此其应用受到限制;由于外侧裂手术通路较为狭窄,因此要求手术医师非常熟悉该区域的解剖结构及毗邻血管走形。
关于颞叶内侧癫痫的诊断、评估、术前准备及手术指证,请参考《颞叶前内侧切除术》章节。
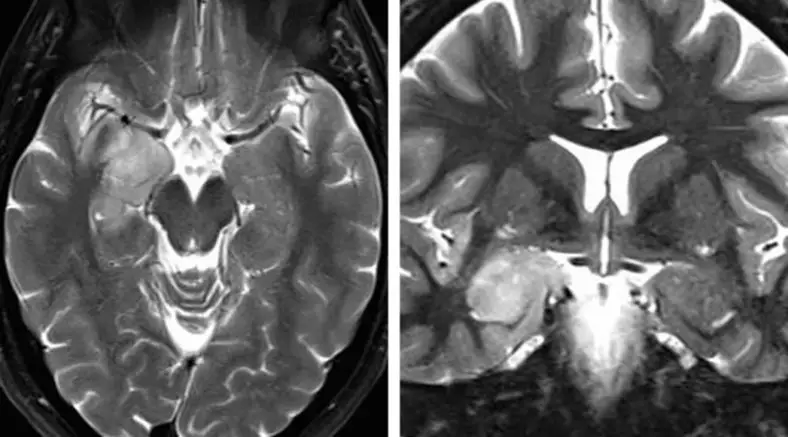
图1. 笔者更偏向于应用SA处理位于杏仁核、钩突及海马前部的肿瘤,尤其是优势半球侧;但针对颞叶内侧硬化症,并不认为SA较标准颞叶前内侧、杏仁核-海马切除术有更显著效果。
术区解剖
颞叶内侧解剖结构复杂,只有完全理解结构及毗邻才能避免术中损伤临近重要血管,其最大风险可能损伤大脑中动脉(MCA)分支、间脑/脑干、脉络膜前动脉及动眼神经。
术中暴露侧脑室颞角常使手术医生迷惑,而术中神经导航对此极有帮助。所有可能受损结构的边界应谨记。自MCA至脉络点(大致可定义为脉络膜前动脉进入脑室的部位,即脉络丛最前方)下端的一条假想线,是位于杏仁核下、苍白球上方的可靠界线。一般情况下杏仁核会延伸至基底池水平,即钩回,邻近动眼神经,而其下部形成了颞角顶部前段及侧壁。
通过颞角前部可识别海马头及体部,海马脚形成了颞角内侧界,而其上方与杏仁核后下方相接。海马体组成了颞角内侧底部,并与形成颞角外侧底部的侧副隆起并行。
位于脉络丛(紧邻丘脑)与海马伞之间的脉络裂,构成了颞角内侧壁的后三分之二。打开脉络裂可通至环池——术中应避免此操作。脉络裂是SA术中重要的地标,因为脉络裂内侧的所有组织都归属丘脑,而外侧的所有组织都可能被切除。显露MCA分叉时颞角作用最为重要,其分叉处与脉络丛能使手术医生确认相关解剖。
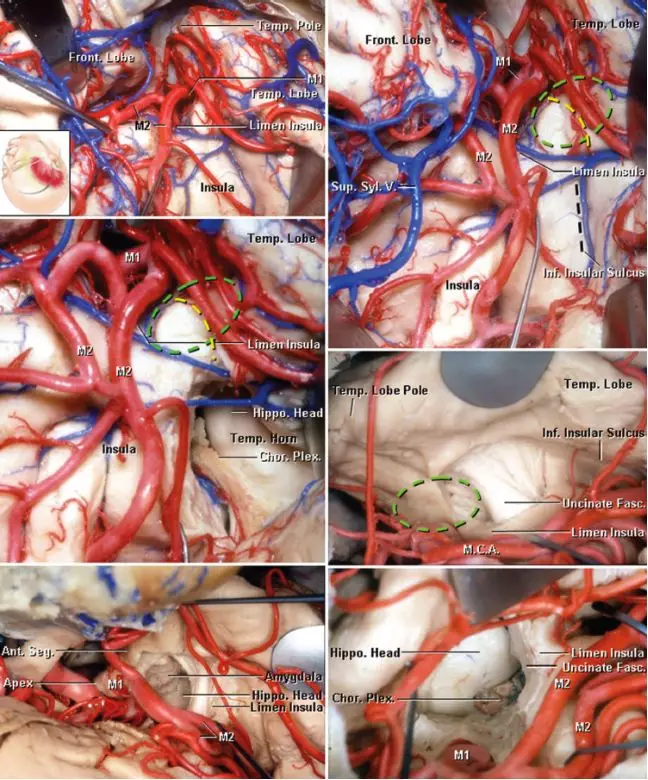
图2. 右侧SA术区解剖。广泛分离外侧裂可见岛阈、岛叶前半部、岛下沟、颞极及MCA的M1、M2段(左上图)。外侧裂外侧被牵开可显示岛阈后方的岛下沟。外侧裂深部的杏仁核用绿线标注。有两种手术入路可到达颞角及杏仁核。黄线直接到达颞角与杏仁核,较位于岛下沟处黑线损伤视辐射风险更小(右上图)。岛下沟处切口可暴露海马头、脉络裂及脉络膜前动脉(左中图)。右中图提供了MCA分叉处分离后的岛阈视野。可经钩束内侧切口切除部分杏仁核,最终颞角处可显露海马头(最下图)(图片由AL Rhoton, Jr授权)。
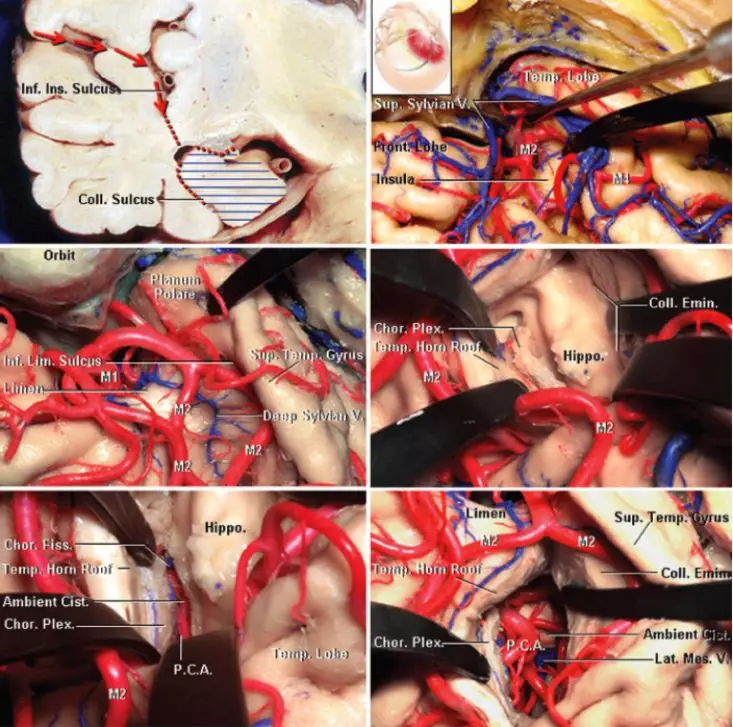
图3. 经右侧颞叶的冠状切口显示了SA的手术路径(左上图)。广泛分离外侧裂(右上图),可见岛下沟前部(界沟)(左中图),经界沟的切口可见颞角、海马、脉络丛、侧副隆起及颞角顶部(右中图)。经脉络膜裂入路,穿过穹隆带、切除海马后可显示基底池内容物(最下图)。(图片由AL Rhoton, Jr授权)
经外侧裂选择性海马-杏仁核切除术
选择性海马-杏仁核切除术有三种常见的入路。第一种是颞下入路,可避免不必要的颞叶外侧皮质损伤;第二种经颞中回皮层入路,技术上较为简单,缺点是会损伤颞叶外侧皮质;第三种即经外侧裂入路,也是笔者偏爱的入路,虽然手术技术上稍困难,但此入路能使手术通路上皮质损伤小,且能保留颞叶外侧皮质结构,相比颞下入路更少对脑组织的牵拉。
Yasargil描述的经外侧裂SA是通过标准翼点开颅完成。患者取仰卧位,头向对侧旋转约30°,直至颧突成为最高点,如此使外侧裂呈垂直方向,即经外侧裂进入颞叶皮质的手术通路中,额叶在重力作用下能够减少对手术的干扰。
打开骨瓣后,磨平眶顶、磨除蝶骨大小翼至眶上裂水平,如此将会减少对额颞叶组织的牵拉,为经外侧裂手术通路提供更为便捷的操作角度。
外侧裂的解剖会在《外侧裂分离技巧》章节中做具体描述。术中需注意尽可能减少对MCA分支的干扰,以降低术后脑血管痉挛的风险;必要时可应用经罂粟碱浸泡的明胶海绵覆盖以减轻脑血管痉挛。
硬膜下操作
接下来分步骤描述SA的硬膜下手术操作技巧。
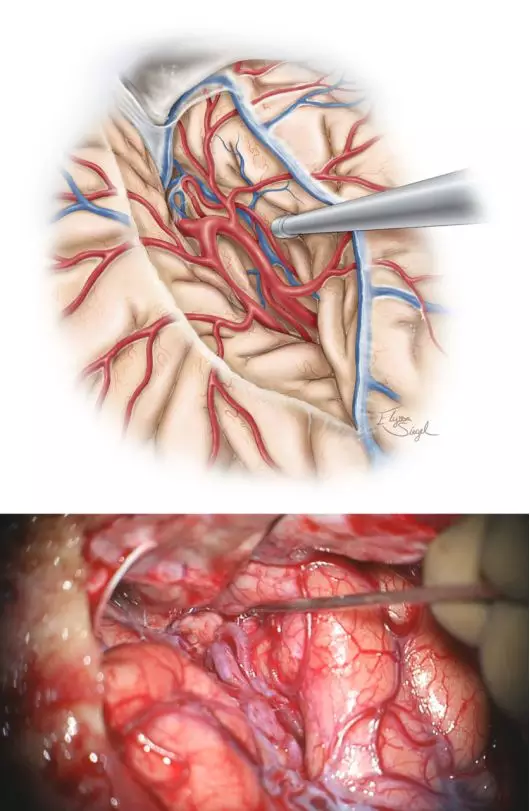
图4. 术中额颞叶应该保持最小的牵拉程度,避免使用过硬的牵拉器,谨防牵拉损伤及静脉栓塞。可应用手动牵引及吸引器行蛛网膜广泛分离,分离外侧裂直至显露颞叶、岛叶及岛下沟与颞干伴行的静脉。
切除海马-杏仁核
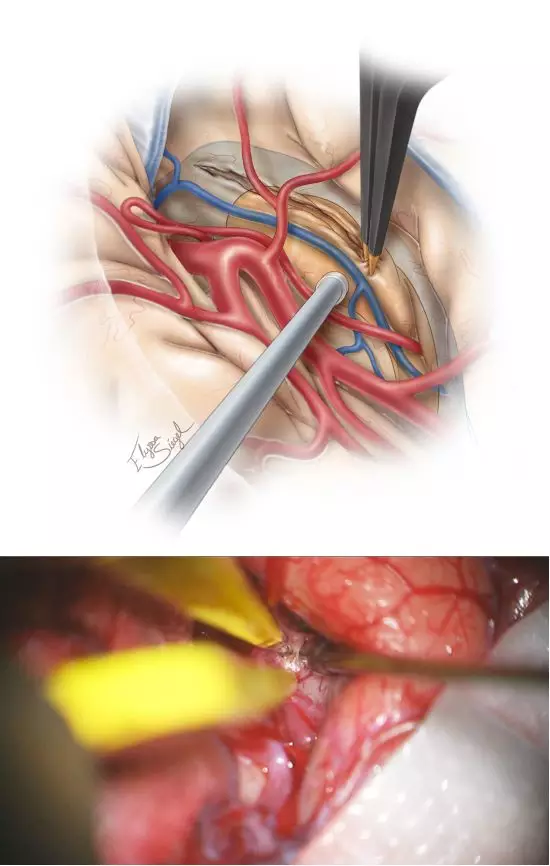
图5. 下一步识别M1及M2近端分支,同时分离显露出岛下部及颞干。笔者在岛下沟的皮层做最初切口(10-20mm),起始部后方或周围见颞极动脉、旁外侧裂下静脉,电凝处理之。此切口也需要术中导航,可标注颞角及海马的大致位置(上图)。
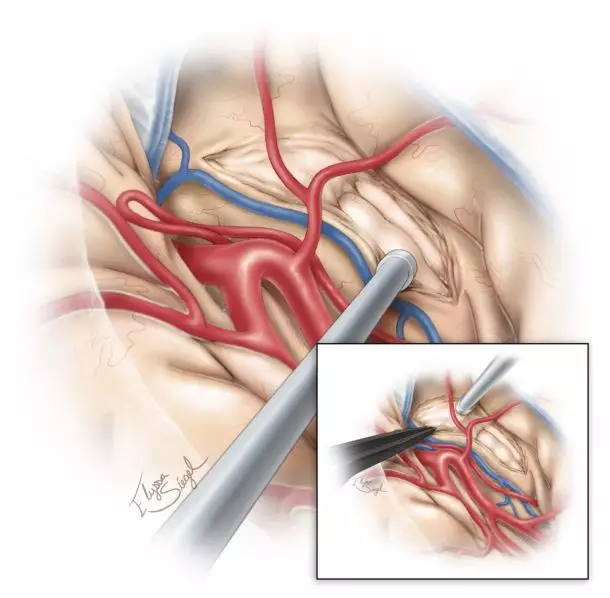
图6. 切开颞干后进入颞角,形成了可探及海马及杏仁核的视野通道。由于之前脑脊液释放后使脑室塌陷,颞角不一定能立即清晰显露。斜面的脑白质容易使术者困惑,因此常规应用神经导航引导下分离此处及切除杏仁核外侧以暴露前颞角。
一般来说,杏仁核向基底池(视交叉池、脚间池与周围)及钩突延伸,钩突分为几段,半月回(钩突前上部)代表着皮层至杏仁核的投射,而边内回(钩突后半部)代表着皮层至海马的投射。半月回由嗅沟与视束从前穿质区分出来,它的侧缘由岛阈形成。
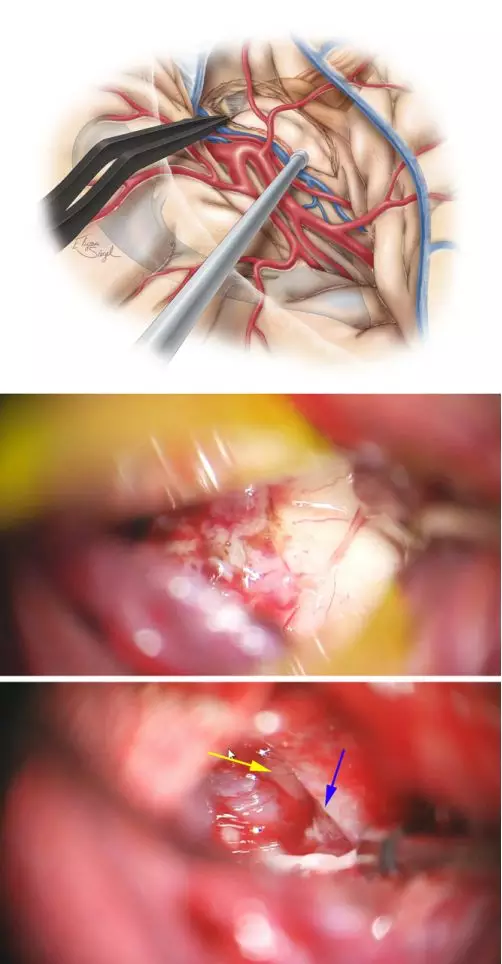
图7. 应用超声刀、双极电凝及吸引器仔细完整切除杏仁核。该法可在残留蛛网膜中可见海马(中图)、小脑幕内缘(蓝色箭头)及动眼神经(黄色箭头)(下图)。自MCA分叉处至脉络丛前缘的虚线划出了杏仁核的上缘。
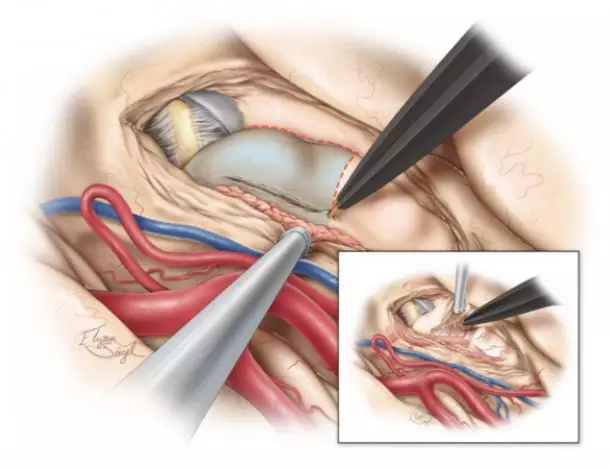
图8. 安全分离海马前部(海马头),自后向前切除。第二步切除海马体、尾部,自前向后一条深在的手术路径可抵到海马后部。
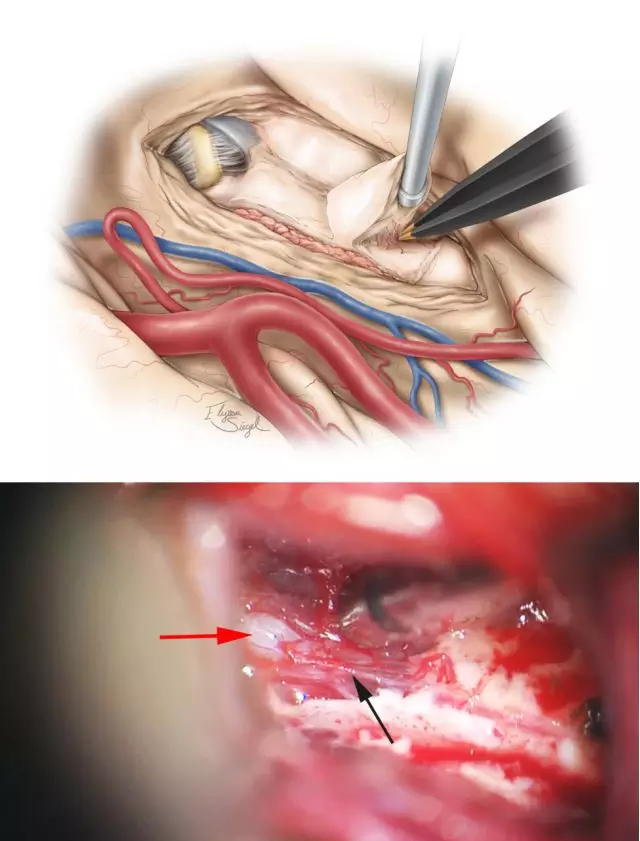
图9. 切除海马的手术技术与上述切除杏仁核相似。手术在脉络丛外、基底池蛛网膜上方的软脑膜下进行,这些膜层需得到保护,而海马旁回在软脑膜下切除。脉络膜前动脉未暴露,大脑后动脉(PCA)(红箭头)得到了保护,给海马后半部供血的侧副沟内穿支(黑箭头)被分离出来以双极电凝切除,以避免脑组织及血管的撕脱。
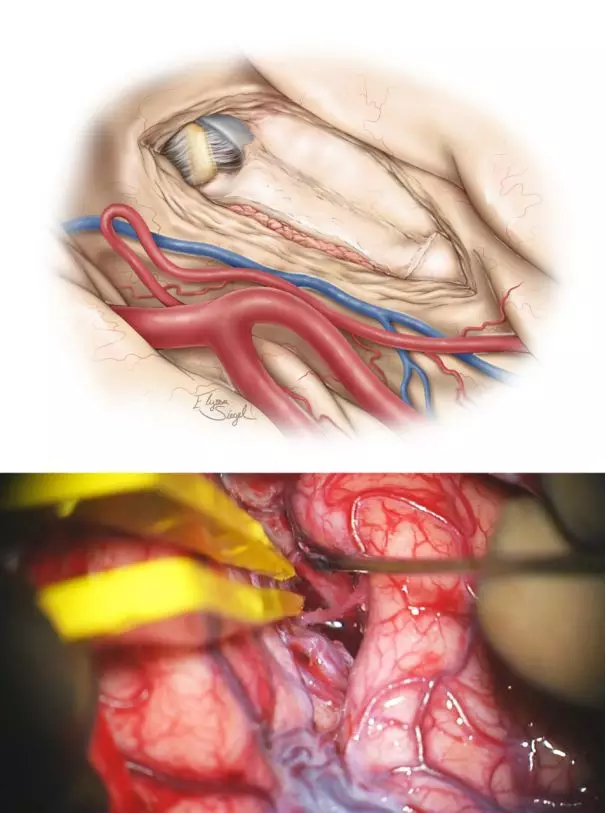
图10. 在脉络丛外侧及基底池蛛网膜层的薄层脑组织被保留下来,避免损伤脑干及间脑组织(上图),术中应注意最小化侵扰术区相关解剖。
SA的手术操作通路虽狭长,但应尽可能避免牵拉或操作引起的MCA分支损伤。显微操作脑压板可动态牵拉;开始外侧裂广泛分离时应避免对额颞叶组织的损伤性牵拉;小脑幕内缘是保护基底池邻近组织的可靠标识;小脑幕边缘蛛网膜层若受到过大牵张力时,可导致滑车神经功能障碍而致短暂术后复视。
可能的并发症
仔细处理外侧裂浅表静脉可减少静脉损伤的风险及随之而来的脑梗死。此处常需要广泛显微分离外侧裂和牵拉MCA的颞叶主干部位,以便拥有在岛旁下沟做皮层切口进入脑室的宽阔手术操作空间。如果未能严格遵守显微手术操作原则,可能会带来灾难性并发症:过度牵拉优势半球侧额颞叶脑盖可致语言障碍;起源于MCA主干的穿支血管受到过度张力时也会受到损伤。
脑血管痉挛:脑血管痉挛是SA术后最常讨论的并发症之一,主要源于术区蛛网膜下腔出血与伴随损伤性的血管操作。在复杂的颞叶解剖中操作,外科医师的手术技术要求对于减少出血、保护血管及减少牵拉损伤至关重要。
视野缺损:与ALT相似,SA后视野缺损较常见。如上半象限盲来源于脑室外侧颞角顶部的Meyer’s环曲度损伤;外侧裂入路经过岛下沟在岛阈平面或其后方5mm左右做皮层切口将需要最大程度保留通过该区域的视辐射。
如果手术医师对颞叶解剖和显微手术技术不够熟悉,且没有神经导航引导时,可合理选择切除颞前内侧皮层后再行切除内侧其他结构的手术方式。
点睛之笔
SA是切除主要位于杏仁核与海马前部病变并保护正常解剖的有效方法。
编译:王小峰
审校:陈成伟、徐涛
DOI: https://doi.org/10.18791/nsatlas.v7.ch02.2
中文版链接: https://www.medtion.com/atlas/2319.jspx
本章节先前曾以其他文体形式呈现,是下列发表文章内容的一部分:
Kovanda TJ, Tubbs RS, Cohen-Gadol AA. Transsylvian selective amygdalohippocampectomy for treatment of medial temporal lobe epilepsy: surgical technique and operative nuances to avoid complications. Surg Neurol Int. 2014;12;5:133. PMID: 25298915
参考文献
Adada B. Selective amygdalohippocampectomy via the transsylvian approach. Neurosurg Focus. 2008;25:E5.
Arruda F, Cendes F, Andermann F, Dubeau F, Villemure JG, Jones-Gotman M, et al. Mesial atrophy and outcome after amygdalohippocampectomy or temporal lobe removal. Ann Neurol. 1996;40:446-450.
Bate H, Eldridge P, Varma T, Wieshmann UC. The seizure outcome after amygdalohippocampectomy and temporal lobectomy. Eur J Neurol. 2007;14:90-94.
Berg AT, Langfitt J, Shinnar S, Vickrey BG, Sperling MR, Walczak T, et al. How long does it take for partial epilepsy to become intractable? Neurology. 2003;60:186-190.
Bonte FJ, Devous MD Sr, Stokely EM, Homan RW. Single-photon tomographic determination of regional cerebral blood flow in epilepsy. AJNR. Am J Neuroradiol. 1983;4:544-546.
Chelune GJ, Naugle RI, Luders H, Awad IA. Prediction of cognitive change as a function of preoperative ability status among temporal lobectomy patients seen at 6-month follow-up. Neurology. 1991;41:399-404.
Choi C, Rubino PA, Fernandez-Miranda JC, Abe H, Rhoton AL, Jr. Meyer's loop and the optic radiations in the transsylvian approach to the mediobasal temporal lobe. Neurosurgery. 2006;59:ONS228-235; discussion ONS235-236.
Clusmann H, Kral T, Gleissner U, Sassen R, Urbach H, Blumcke I, et al. Analysis of different types of resection for pediatric patients with temporal lobe epilepsy. Neurosurgery. 2004;54:847-859; discussion 859-860.
Clusmann H, Schramm J, Kral T, Helmstaedter C, Ostertun B, Fimmers R, et al. Prognostic factors and outcome after different types of resection for temporal lobe epilepsy. J Neurosurg. 2002;97:1131-1141.
de Tisi J, Bell GS, Peacock JL, McEvoy AW, Harkness WF, Sander JW, et al. The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet. 2011;378:1388-1395.
Devous MD, Sr, Thisted RA, Morgan GF, Leroy RF, Rowe CC. SPECT brain imaging in epilepsy: a meta-analysis. J Nucl Med. 1998;39:285-293.
Dulay MF, Levin HS, York MK, Li X, Mizrahi EM, Goldsmith I, et al. Changes in individual and group spatial and verbal learning characteristics after anterior temporal lobectomy. Epilepsia. 2009;50:1385-1395.
Egan RA, Shults WT, So N, Burchiel K, Kellogg JX, Salinsky M. Visual field deficits in conventional anterior temporal lobectomy versus amygdalohippocampectomy. Neurology. 2000;55:1818-1822.
Engel J, Jr., McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA. 2012;307:922-930.
Engel J, Jr., Wiebe S, French J, Sperling M, Williamson P, Spencer D, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology. 2003;60:538-547.
French JA, Williamson PD, Thadani VM, Darcey TM, Mattson RH, Spencer SS, et al. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol. 1993;34:774-780.
Harvey DJ, Naugle RI, Magleby J, Chapin JS, Najm IM, Bingaman W, et al. Relationship between presurgical memory performance on the Wechsler Memory Scale-III and memory change following temporal resection for treatment of intractable epilepsy. Epilepsy Behav. 2008;13:372-375.
Helmstaedter C, Reuber M, Elger CC. Interaction of cognitive aging and memory deficits related to epilepsy surgery. Ann Neurol. 2002;52:89-94.
Hermann BP, Seidenberg M, Schoenfeld J, Davies K. Neuropsychological characteristics of the syndrome of mesial temporal lobe epilepsy. Arch Neurol. 1997;54:369-376.
Ho SS, Berkovic SF, Berlangieri SU, Newton MR, Egan GF, Tochon-Danguy HJ, et al. Comparison of ictal SPECT and interictal PET in the presurgical evaluation of temporal lobe epilepsy. Ann Neurol. 1995;37:738-745.
Hori T, Tabuchi S, Kurosaki M, Kondo S, Takenobu A, Watanabe T. Subtemporal amygdalohippocampectomy for treating medically intractable temporal lobe epilepsy. Neurosurgery. 1993;33:50-56; discussion 56-57.
Jones-Gotman M, Harnadek MC, Kubu CS. Neuropsychological assessment for temporal lobe epilepsy surgery. Can J Neurol Sci. 2000;27 Suppl 1:S39-43; discussion S50-52.
Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069-1077.
Lackner P, Koppelstaetter F, Ploner P, Sojer M, Dobesberger J, Walser G, et al. Cerebral vasospasm following temporal lobe epilepsy surgery. Neurology. 2012;78:1215-1220.
Mansouri A, Fallah A, Valiante TA. Determining surgical candidacy in temporal lobe epilepsy. Epilepsy Res Treat. 2012;2012:706917.
Mengesha T, Abu-Ata M, Haas KF, Lavin PJ, Sun DA, Konrad PE, et al. Visual field defects after selective amygdalohippocampectomy and standard temporal lobectomy. J Neuroophthal. 2009;29:208-213.
Mohammed HS, Kaufman CB, Limbrick DD, Steger-May K, Grubb RL, Jr., Rothman SM, et al. Impact of epilepsy surgery on seizure control and quality of life: a 26-year follow-up study. Epilepsia. 2012;53:712-720.
Morino M, Uda T, Naito K, Yoshimura M, Ishibashi K, Goto T, et al. Comparison of neuropsychological outcomes after selective amygdalohippocampectomy versus anterior temporal lobectomy. Epilepsy Behav. 2006;9:95-100.
Paglioli E, Palmini A, Portuguez M, Paglioli E, Azambuja N, da Costa JC, et al. Seizure and memory outcome following temporal lobe surgery: selective compared with nonselective approaches for hippocampal sclerosis. J Neurosurg. 2006;104:70-78.
Park TS, Bourgeois BF, Silbergeld DL, Dodson WE. Subtemporal transparahippocampal amygdalohippocampectomy for surgical treatment of mesial temporal lobe epilepsy. Technical note. J Neurosurg. 1996;85:1172-1176.
Radhakrishnan K, So EL, Silbert PL, Jack CR, Jr., Cascino GD, Sharbrough FW, et al. Predictors of outcome of anterior temporal lobectomy for intractable epilepsy: a multivariate study. Neurology. 1998;51:465-471.
Rausch R, Babb TL. Hippocampal neuron loss and memory scores before and after temporal lobe surgery for epilepsy. Arch Neurol 1993;50:812-817.
Rosenow F, Luders H. Presurgical evaluation of epilepsy. Brain. 2001;124:1683-1700.
Sagher O, Thawani JP, Etame AB, Gomez-Hassan DM. Seizure outcomes and mesial resection volumes following selective amygdalohippocampectomy and temporal lobectomy. Neurosurg Focus. 2012;32:E8.
Schaller C, Jung A, Clusmann H, Schramm J, Meyer B. Rate of vasospasm following the transsylvian versus transcortical approach for selective amygdalohippocampectomy. Neurol Res. 2004;26:666-670.
Schaller C, Zentner J. Vasospastic reactions in response to the transsylvian approach. Surg Neurol. 1998;49:170-175.
Semah F, Picot MC, Adam C, Broglin D, Arzimanoglou A, Bazin B, et al. Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology. 1998;51:1256-1262.
Takaya S, Mikuni N, Mitsueda T, Satow T, Taki J, Kinoshita M, et al. Improved cerebral function in mesial temporal lobe epilepsy after subtemporal amygdalohippocampectomy. Brain. 2009;132:185-194.
Tanriverdi T, Olivier A. Cognitive changes after unilateral cortico-amygdalohippocampectomy unilateral selective-amygdalohippocampectomy mesial temporal lobe epilepsy. Turk Neurosurg. 2007;17:91-99.
Tubbs RS, Miller JH, Cohen-Gadol AA, Spencer DD. Intraoperative anatomic landmarks for resection of the amygdala during medial temporal lobe surgery. Neurosurgery. 2010;66:974-977.
Wen HT, Rhoton AL, Jr, de Oliveira E, Cardoso AC, Tedeschi H, Baccanelli M, et al. Microsurgical anatomy of the temporal lobe: part 1: mesial temporal lobe anatomy and its vascular relationships as applied to amygdalohippocampectomy. Neurosurgery. 1999;45:549-591; discussion 591-592.
Wendling AS, Hirsch E, Wisniewski I, Davanture C, Ofer I, Zentner J, et al. Selective amygdalohippocampectomy versus standard temporal lobectomy in patients with mesial temporal lobe epilepsy and unilateral hippocampal sclerosis. Epilep Res. 2013;104:94-104.
Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. New Engl J Med. 2001;345:311-318.
Wieser HG, Ortega M, Friedman A, Yonekawa Y. Long-term seizure outcomes following amygdalohippocampectomy. J Neurosurg. 2003;98:751-763.
Yasargil MG, Krayenbuhl N, Roth P, Hsu SP, Yasargil DC. The selective amygdalohippocampectomy for intractable temporal limbic seizures. J Neurosurg. 2010;112:168-185.
Zubal IG, Spencer SS, Imam K, Seibyl J, Smith EO, Wisniewski G, et al. Difference images calculated from ictal and interictal technetium-99m-HMPAO SPECT scans of epilepsy. J Nucl Med. 1995;36:684-689.
相关链接



